Brian J.F. Wong, M.D., PhD Research Lab
Subglottic stenosis (SGS) is described as a narrowing of the airway and is the most common abnormality/acquired anomaly in newborns and infants. Surgical endoscopy (placing a camera down the patient’s throat) has remained the gold standard in the evaluation of the newborn suspected of subglottic stenosis. However, surgical endoscopy can cause additional trauma to the airway that can lead to edema (tissue swelling) and scarring of airway tissue. Early diagnosis of disease progression would allow early aggressive pharmacologic treatment and optimized airway management in the neonatal intensive care unit (NICU). Early intervention can thus reduce and/or eliminate the need for diagnostic surgical endoscopy and major airway reconstructive surgery. Our goal is to design and construct Optical Coherence Tomography (OCT) instrumentation to image the newborn and infant airways and define the potential role of OCT in diagnosing the onset and progression of subglottic airway disease in these critically ill patients.
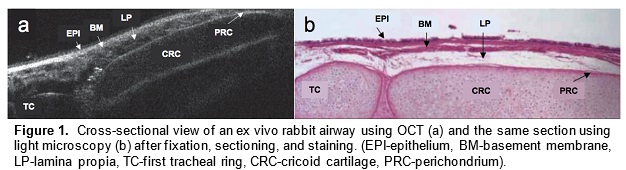
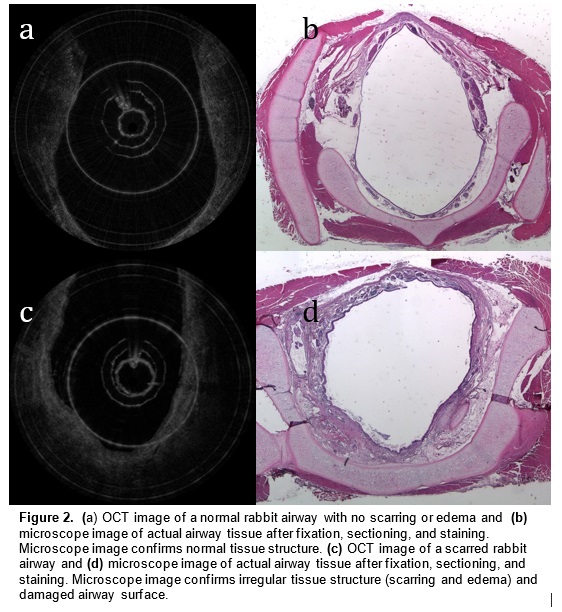
Optical coherence tomography (OCT) is an imaging technology that utilizes long wavelength light to penetrate tissue and create high-resolution cross-sectional images. We are developing a specialized, long-range OCT device that focuses on the shapes of hollow systems like the airway. OCT imaging is less invasive than surgical endoscopy and thus reduces the risk of further damaging the airway. Another key advantage of OCT over other imaging technologies is its ability to penetrate different mediums and image subsurface tissue layers. It can also provide vivid images of the subglottic airway tissue microstructure. A pilot study demonstrated that OCT is able to image and distinguish scarring or edema in airway tissue. Providing neonatologists with this anatomic information will enable better management and treatment of subglottic airway disease.
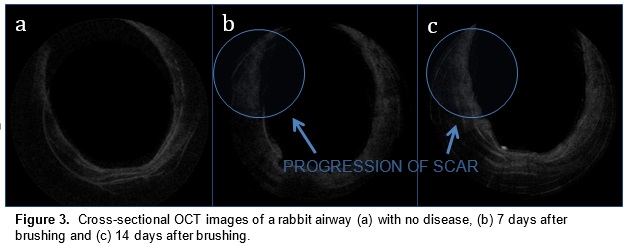
In a preliminary study, we have mapped subglottic airways in several rabbit subjects using the OCT system. For each rabbit, a plastic brush was used to score and abrade the subglottic mucosa, creating injuries that would eventually create fibrosis and scar. This procedure creates a circumferential injury that causes the airway to narrow over time. The rabbits were closely monitored and imaged at regular time intervals using the OCT system following the initial brushing in order to track changes in tissue structure and progression of disease. OCT imaging created a series of 2 dimensional axial representations of the subglottic airway similar to CT/MRI imaging output. Our goal is to correlate OCT images with histology in order to gain information on how injury to the airway mucosa evolves and how edema, granulation, and scar appear in the airway. Detailed analysis will be performed on the dimensions of key morphologic features (the thickness of different tissue layers, the thickness of tracheal and cricoid cartilages) with the aim of finding structural features that correlate with specific diagnoses (scarring, edema, stenosis).
The next step is to image neonates at UC Irvine Medical Center (UCIMC) and the Children’s Hospital of Orange County (CHOC). Imaging will involve the insertion of the low profile imaging probe/catheter through the endotracheal tube to image the entire larynx and proximal trachea. Dr. Gurpreet Ahuja is the director pediatric otolaryngology at both institutions and will work alongside Dr. Brian Wong in patient selection, imaging, and data analysis. We hope to characterize the tissue micro architecture of intubated neonatal ICU patients and correlate OCT findings with clinical metrics and results from surgical endoscopy, with the broad aim of developing OCT as a means of diagnosing subglottic airway changes and monitoring the acute progression of disease.
Upper airway obstruction affects up to 3% of all children. It is most commonly caused by enlarged tonsils and adenoids (adenotonsillar hypertrophy). The removal of these, adenotonsillectomy (AT), is among the most common operations performed in the United States. While AT significantly relieves symptoms in the vast majority of children, it does not benefit many others, and this is particularly true in children with craniofacial anomalies, Down’s syndrome, and obesity. Identifying children who will fail to respond to AT prior to surgery is exceptionally challenging. To ultimately aid physicians in this process, we are developing technology to image and measure the upper airway in patients, as well as a computational model to simulate airflow in the upper airway. Together these can be used to obtain information that will improve the accuracy of both selecting patients for surgery and predicting their response to it.
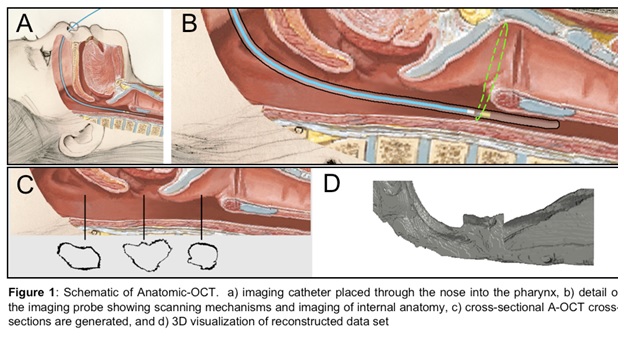
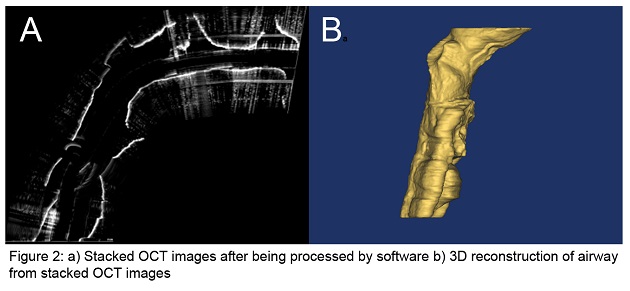
Optical Coherence Tomography (OCT) is a novel imaging technology we are developing to obtain structural data on the pediatric upper airway. While no currently available diagnostic tests can provide complete volumetric information of the airway in real-time for sustained time intervals (e.g. overnight), OCT can potentially address this need relatively inexpensively, without the need for sedation, and in an office setting. This is in contrast to currently used imaging technologies such as fast computed tomography (CT) and cine magnetic resonance imaging (MRI), which are costly, require sedation, and in the case of CT, expose the child to ionizing radiation. OCT works similarly to ultrasound but uses light rather than sound to generate structural information on turbid media based upon differences in optical absorption and scattering. An offshoot of conventional OCT, anatomic OCT (A-OCT) can be used to determine the size and shape of various hollow organs such as the upper airway. In A-OCT of the upper airway (see Figure 1), an optical fiber is placed within a slender, soft transparent tube (~3mm), which is then advanced through the nostrils and then positioned into the esophagus for stabilization. The fiber can be simultaneously rotated and withdrawn to generate a helical scan of the patient’s upper airway anatomy. The data can then be used to reconstruct the airway anatomy (Figure 2). We seek to develop a high speed, long range A-OCT system by narrowing its linewidth and incorporating nanotechnology for real-time imaging processing and 3-D reconstruction and visualization.
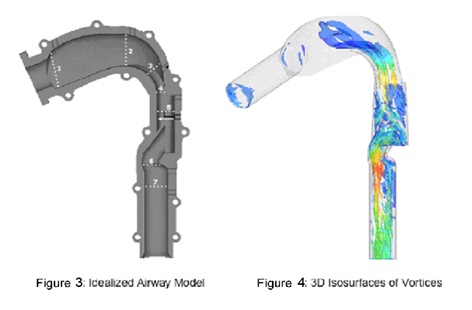
We will use the size and shape of the upper airway determined by high speed A-OCT in conjunction with a numerical model of airflow through the airway in order to determine areas of obstruction. Currently, structural data is obtained from high speed CT and cine MRI for use with a model based on computational fluid dynamics (CFD). In addition to the disadvantages to the use of CT and cine MRI listed above, additional drawbacks of this strategy are the limitations of commonly-used CFD, which has limited accuracy modeling transitional and turbulent flow in the airway. Rather than commonly-used CFD, we will be using Direct Numerical Simulation (DNS) run by supercomputers to simulate flow in the upper airway, as it can overcome these challenges. DNS provides detailed and highly accurate information on the flow of air and pressure, which is required for a comprehensive understanding of airway behavior and is essential to determining and identifying regions of potential airway collapse (Figures 3 and 4).
By using a DNS model with high speed A-OCT imaging technology, we will be able to image the upper airway anatomy in children and then simulate the flow of air in the upper airway to gain information on flow, pressure, and turbulence. This information will be used to compare patients with and without adenotonsillar hypertrophy, as well as patients with adenotonsillar hypertrophy before and after surgery to evaluate the clinical sensitivity of the DNS/A-OCT technology. Ultimately, the DNS/A-OCT technology will be used to select patients for surgery and predict their outcomes. The structural information on internal airway anatomy will allow simulation of upper airway airflow and estimation of the impact of surgery on relieving airway obstruction. In turn, the model will provide a means to determine which children will benefit from upper airway surgery, and is a first step toward developing individualized surgical therapy.
Research Awards
Principal Investigator(s):
Zhongping Chen, PhD
Brian Wong, MD
Project Title: In vivo Imaging and Quantification of Cilia Beating Dynamics Using Phase-
Resolved Optical Imaging Technology
Total award: $2,367,511

